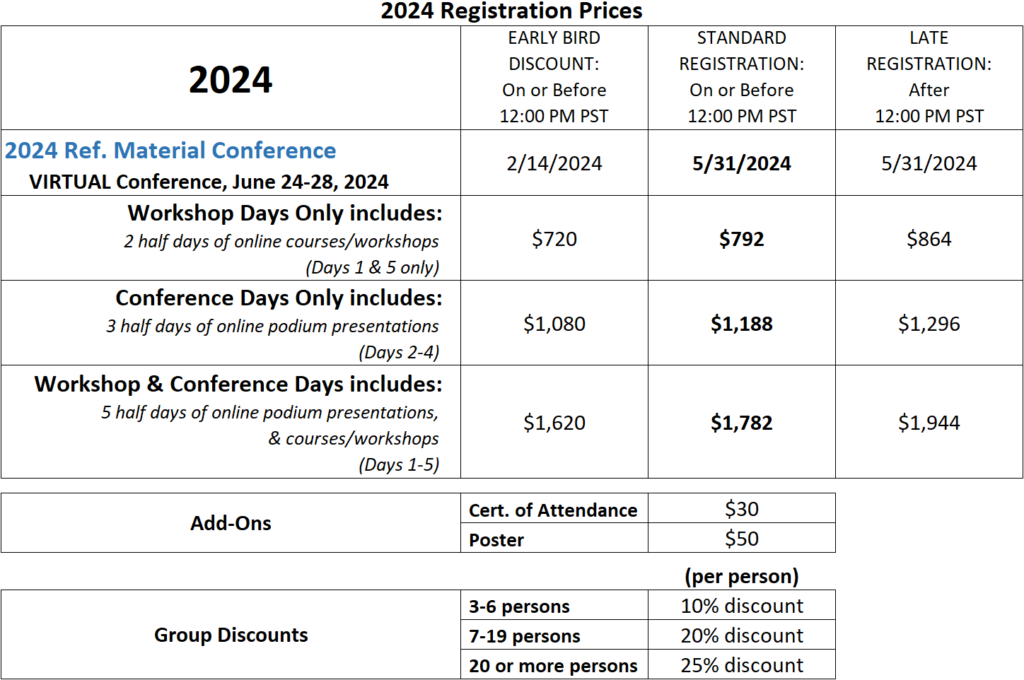
This BEBPA conference include 5 half-days of presentations, workshops and round-table discussions on topics of current interest to the analytical biopharmaceutical community. It is hoped that these presentations and discussions will provide topics for future white paper working groups.
2024 Session Topics
- Challenges With The First Relative Potency IRM
- Potency IRM Bridging and Stability Challenges
- Relative Potency IRM Challenges New Modalities
2024 Workshops
- Overview of Reference Material Programs
- Cell & Gene Therapy Reference Standards
- Biosimilar Reference Standards
Agenda
Download WHOVA before the conference to be able to view the conference agenda, speaker bios and engage with the BEBPA community!
2024 RFM Speaker Abstracts
Title: mAb Case Study on Challenges for Bridging Reference Standard Potency during Clinical Phases and Monitoring of its Stability
Abstract: As part reference standard (RS) replacement process, from the interim to the first GMP (RS) or later during development or commercial phase, the bridging of potency raises several challenges to avoid shift or drift in batch release. The following topics will be discussed considering also recent feedbacks from various health authorities:
- Testing design (sample size) to ensure relevant potency determination considering the method variability
- Acceptance criteria for relative potency
- Impact of change of method for relative potency determination (eg from binding ELISA test to a Cell-based assay)
In addition, monitoring the stability of a reference standard in term of its relative potency over its life cycle raises also challenges and concerns from the health authorities
Title: Design and Preparation of Reference Materials to Standardise Biological Activity Measurements
Abstract: To Be Announced
Title: An Overview of Analytical Development Activities for Establishing and Maintaining Biologics Reference Standards
Abstract: Biologic reference standards have especial considerations and practices that differ from those applied to small molecule programs, most notably how they are used in relative potency bioassays. In connection with an initial fusion protein development program, the Analytical Development department is tasked with building internal policies and procedures to establish and maintain biological reference standards in support of an oncology pipeline. These reference standards are intended to be implemented at the pre-clinical stage and the type and amount of supportive data grows in complexity parallel to the drug lifecycle during clinical development. The primary biological reference standard, to be used after commercialization of the drug, needs to be traced back to the original standard qualified during pre-clinical development. It is particularly challenging to maintain alignment of the relative potency reference standards throughout development because the process and the potency assay(s) continue to be improved. This presentation provides an overview of the types of analytical activities in place for establishing, bridging, and monitoring the stability of product reference standards necessary to support an internal platform pipeline.
Title: Building a Foundation for Success: Establishment and Management of Relative Potency Reference Material during Early Development
Abstract: To Be Announced
Title: Connecting the Dots: Primary and Working Reference Standards for Allogeneic CAR T Cells
Abstract: The strategies for design and use of potency assays for Cell and Gene Therapeutics is a developing story. Originally most of these assays were based upon ‘absolute’ potency. More recently, they have begun to transition to ‘relative’ potency. That shift to relative potency assays is associated with the concurrent need for a reference standard. While the desire and intention are to adopt a classical Primary & Working Reference Standard approach, the support of a cellular allogenic CAR-T therapeutic brings with it unique challenges, including donor-to-donor variability, limited lots sizes, and more. We will discuss a possible solution we have undertaken to address these challenges.
Title: Biosimilar Reference Standards
Abstract: To Be Announced